Safety data collection tools for real-world reporting
Description
Resources for the collection of safety data by non-clinician and clinician data collectors
Category: Intervention Manuals
Sub Category: Intervention Manuals
Author: Cheryl Pace
Published Date: 23 May 2014
Description
[Français]
How can we assess the safety of antimalarials? from ACT Consortium on Vimeo.
Adverse event monitoring is traditionally conducted by clinicians in healthcare facilities. However, as treatment is increasingly being accessed at the community level, it is important that providers of treatment in the community are given the appropriate tools to monitor for adverse events in their patients, to determine if the medicine is causing them any ill-effects.
These safety data collection tools were developed to enable Community Healthcare Workers (CHWs) and other non-clinicians to collect adverse event data from patients who have been administered anti-malarials.
Unlike conventional adverse event monitoring tools which can be complex, these forms are designed for use in lower-literacy settings. They utilise pictures to convey the purpose of the data collection to the patient, and a diary for capturing patient-reported events that have occurred in the follow up period and details of medication taken.
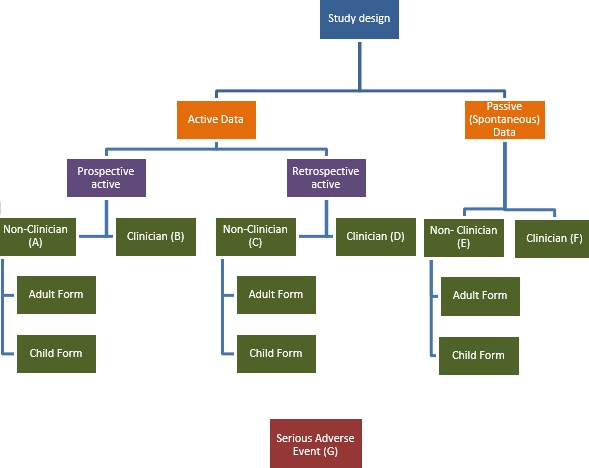
A training manual has been developed to provide detailed guidance on the use of these forms, including a step-by-step guide to completing the forms followed by a quick-guide, designed to be carried in the field and referred to at the time of data collection. Each chapter refers to a form which has been tailored to suit the context of data collection.
Clinician forms
Forms for use by clinicians have also been developed and are available to download at the bottom of this page. Accompanying Standard Operating Procedures will follow shortly.
Please refer to the diagram and introduction in the manual to decide which form is most suitable for your needs.
To read more about the development of these tools, please refer to:
Davies EC, Chandler CIR, Innocent SHS, Kalumuna C, Terlouw DJ, et al. (2012) Designing Adverse Event Forms for Real-World Reporting: Participatory Research in Uganda. PLoS ONE 7(3): e32704. doi:10.1371/journal.pone.0032704
Whilst these tools were developed by the ACT Consortium for use within malaria studies, they are transferable to other disease-contexts and programmatic settings; please refer to the usage policy before downloading.


